Ever wonder what life at Clark is like? The five video series I created attempts to answer the beginning of those sort of questions.
Why should students pick your major?
What was your favorite class at Clark?
What is your favorite restaurant close to campus?
What is your favorite campus event?
What is the one thing you look forward to all year?
Friday, July 29, 2011
Monday, July 18, 2011
General campus lolz
My girlfriend and I had dinner on the green the other night, and it was really pretty. But then this squirrel started getting a little close and started to creep us out.
This is after I posted this video to my YouTube channel:
On an unrelated note, I made this Rage Comic last week, which I think sums up my summer pretty well:
Also, more dry ice:
 |
| My little friend got a little close for comfort. |
This is after I posted this video to my YouTube channel:
On an unrelated note, I made this Rage Comic last week, which I think sums up my summer pretty well:
Also, more dry ice:
Sunday, July 10, 2011
How to reuse plastic pipet tip loaders
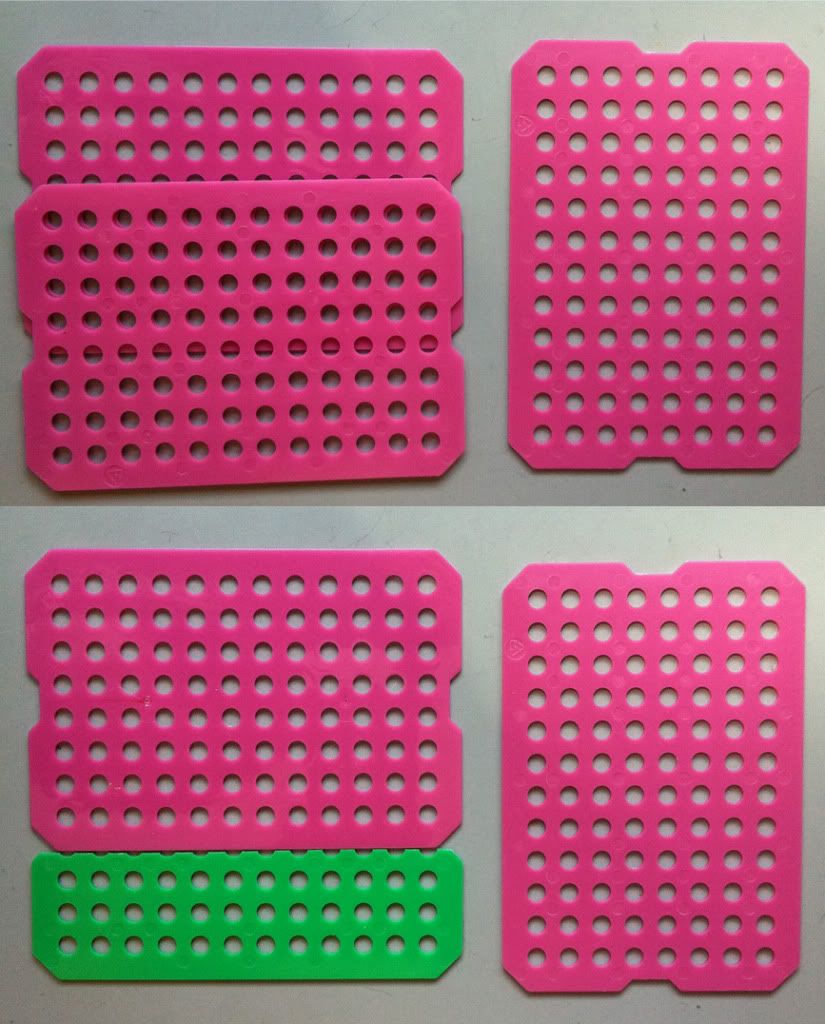
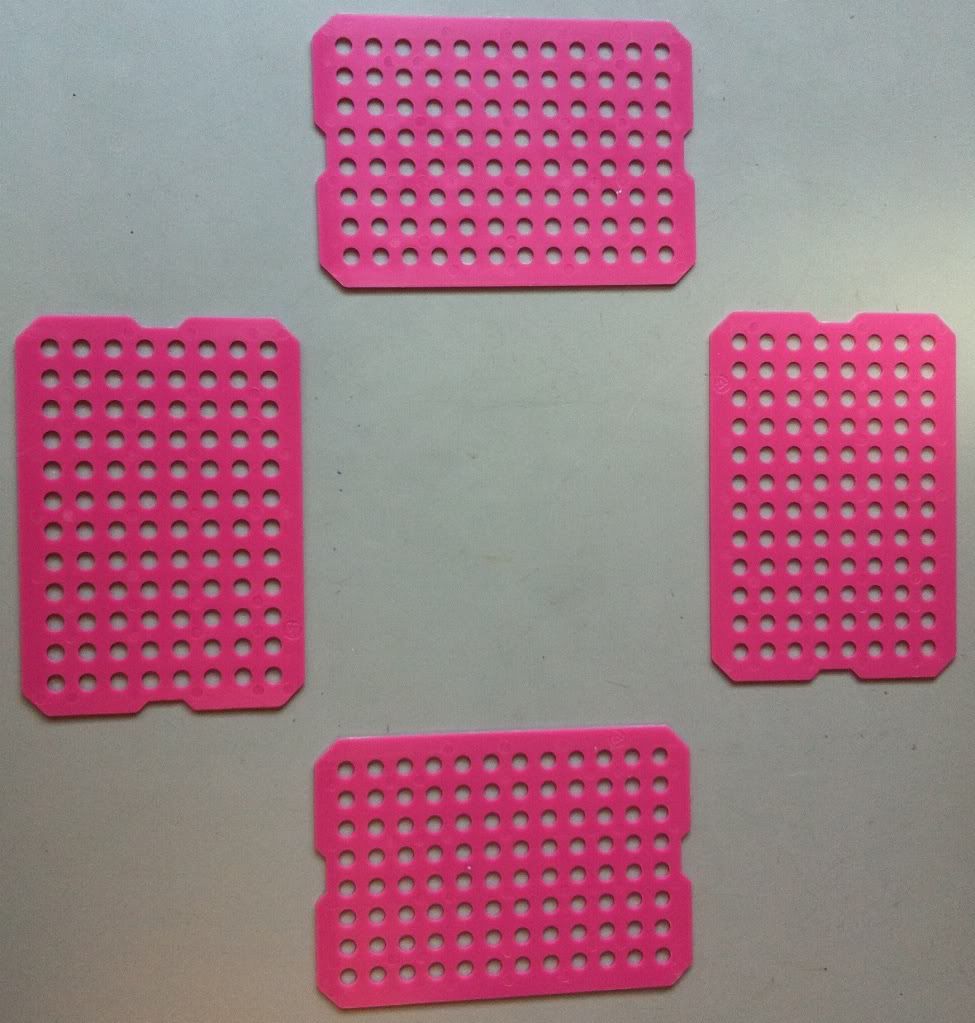
Working in a biology lab, you may have discovered the wonders of boxed pipet tip packs that allow you to quickly load multiple boxes of pipet tips. These boxes of pipet tips are made up of multiple layers of tips stacked on top of each other, separated by plastic sheets (below), which are left behind once you finish a box of pipet tips. I've been collecting these sheets for quite a while and have since found a use for them.
With some ordinary lab tape, these plastic sheets can be made into handy baskets used to separate lab supplies. By stacking and taping the sheets in different orientations, you can make custom shaped baskets that accommodate tubes, caps, or anything else your lab has generous quantities of.
At first, I made dividers--a square of sheets taped together--that allowed the separation of caps from tubes in our messy lab tube drawer. While this made the messy lab tube drawer a little tidier, I found that making the dividers into baskets (by giving them a bottom) greatly increased their efficiency because I could pick up and shuffle the order of the tubes and caps within the drawer.
These plastic sheets can be made into a wide variety of different shapes and sizes of baskets. Overlapping the plastic sheets or carefully cutting off a section of a sheet allows you to customize a basket to fit those awkwardly shaped tubes or hold your collection of caps that has gotten out of control.
While you could probably figure it out yourself now that I've given you this awesome idea, and there are definitely a ton of ways to make these baskets, below is a general step-by-step guide to make these crafty creations.
Supplies needed in order to create these crafty creations:
1.) Six or more plastic pipet tip loader sheets.
2.) Tape (I used lab tape because it is handy, fairly strong, and comes in a variety of colours).
3.) A cutting device (most people use scissors... which I recommend. Razor blades cut thumbs, but of course I wouldn't know that first hand).
Directions:
1.) Decide the size of the basket you need. I'm making a basic 1 by 1 sheet basket to hold tube caps.
2.) Because these sheets are rectangles, the bottom of the basket will most likely have to be cut and taped together to make a square. I do one of two things:
Top: you can overlap two sheets to match the length of the basket side.
Bottom: you can trim the second sheet to match the length of the basket side, so the bottom of the basket will lay flush instead of having a slight bump.
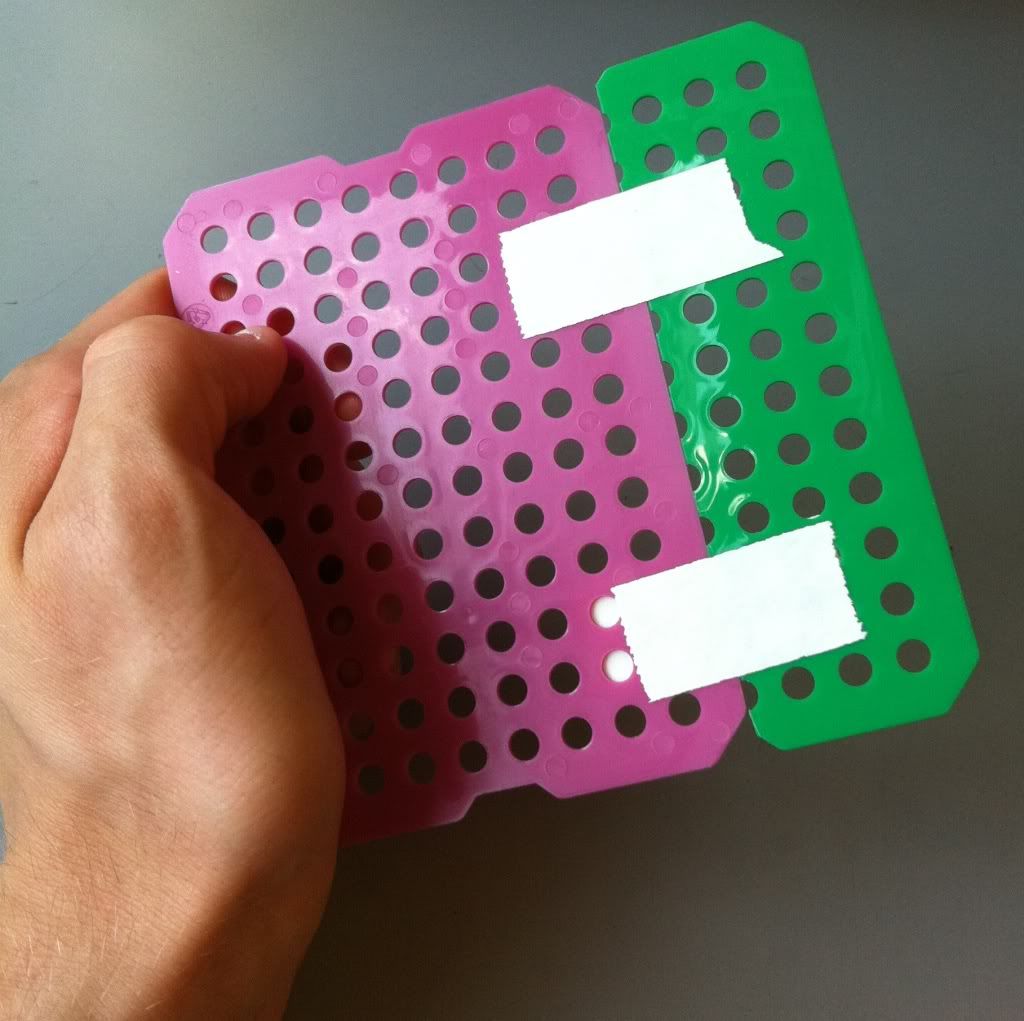
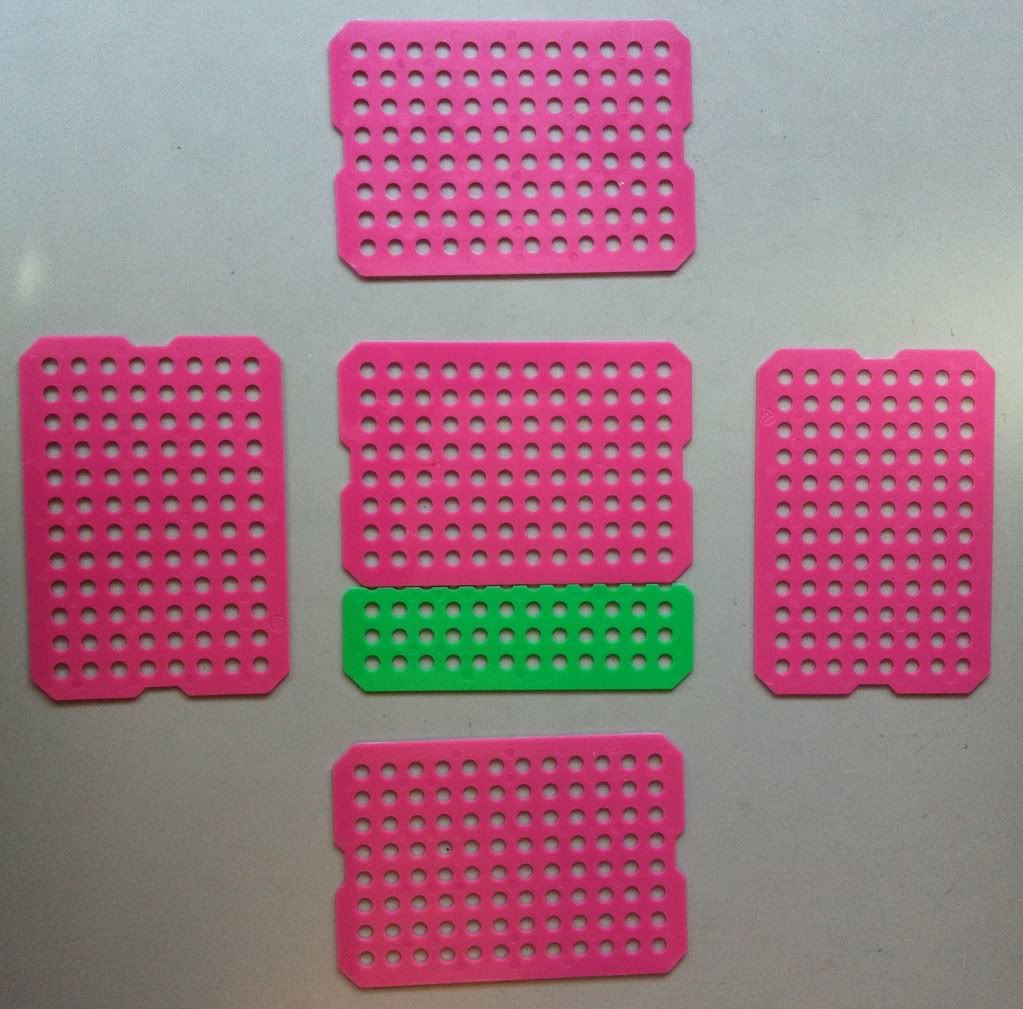
I'm going to go the second route and use the spare green piece of plastic sheet to make my bottom. The top option (overlapping sheets) is stronger, but won't sit evenly on a flat surface.
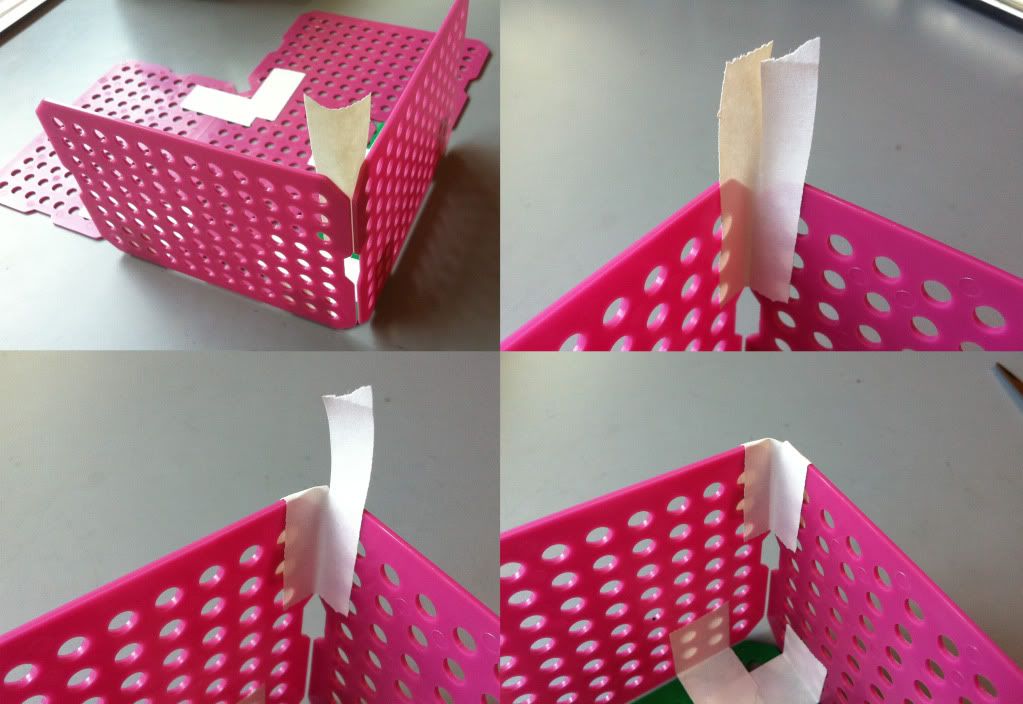
3.) Tape the basket bottom together. Be sure to put tape on either side of the sheets.
Then place a piece of tape on the inside of the sides where they meet. Cut the tape from the top down to the edge of the basket and fold the pieces of tape over. Repeat this step by placing a piece of tape on the outside of the basket sides, cut the tape, and fold the pieces of the tape toward the inside of the basket. Both the inside and the outside of the basket corner top should be covered in tape.
 |
| Plastic tip loader sheets, henceforth called plastic sheets. They need a better name. |
With some ordinary lab tape, these plastic sheets can be made into handy baskets used to separate lab supplies. By stacking and taping the sheets in different orientations, you can make custom shaped baskets that accommodate tubes, caps, or anything else your lab has generous quantities of.
At first, I made dividers--a square of sheets taped together--that allowed the separation of caps from tubes in our messy lab tube drawer. While this made the messy lab tube drawer a little tidier, I found that making the dividers into baskets (by giving them a bottom) greatly increased their efficiency because I could pick up and shuffle the order of the tubes and caps within the drawer.
These plastic sheets can be made into a wide variety of different shapes and sizes of baskets. Overlapping the plastic sheets or carefully cutting off a section of a sheet allows you to customize a basket to fit those awkwardly shaped tubes or hold your collection of caps that has gotten out of control.

While you could probably figure it out yourself now that I've given you this awesome idea, and there are definitely a ton of ways to make these baskets, below is a general step-by-step guide to make these crafty creations.
Supplies needed in order to create these crafty creations:
1.) Six or more plastic pipet tip loader sheets.
2.) Tape (I used lab tape because it is handy, fairly strong, and comes in a variety of colours).
3.) A cutting device (most people use scissors... which I recommend. Razor blades cut thumbs, but of course I wouldn't know that first hand).
Directions:
1.) Decide the size of the basket you need. I'm making a basic 1 by 1 sheet basket to hold tube caps.
 |
| One sheet by one sheet (these will be the side of the basket). |
Top: you can overlap two sheets to match the length of the basket side.
Bottom: you can trim the second sheet to match the length of the basket side, so the bottom of the basket will lay flush instead of having a slight bump.
I'm going to go the second route and use the spare green piece of plastic sheet to make my bottom. The top option (overlapping sheets) is stronger, but won't sit evenly on a flat surface.
 |
| With the bottom of the basket sized up, it's time to start taping this bad boy together. Professor! Where do we keep our tape? |
3.) Tape the basket bottom together. Be sure to put tape on either side of the sheets.
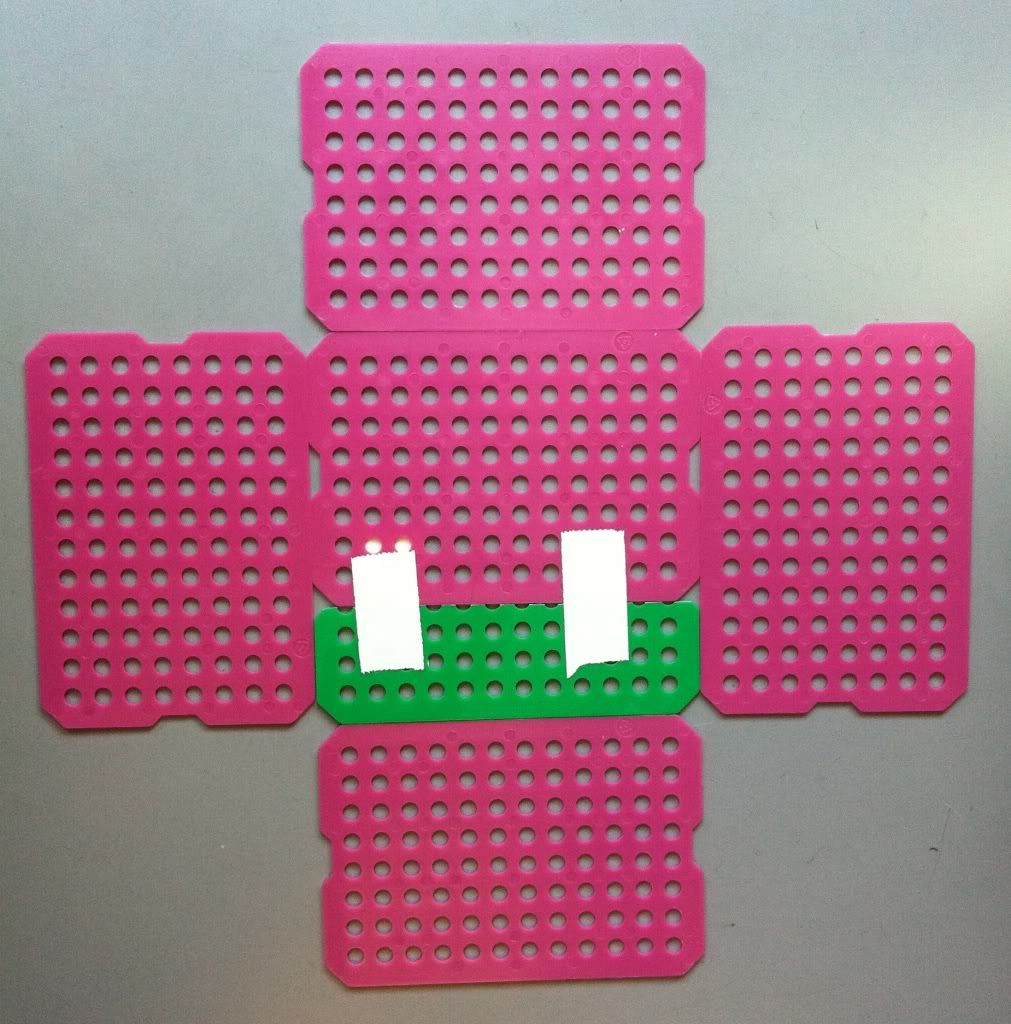
4.) Tape the four sides of the basket onto the bottom. I do NOT tape the outside of the bottom to each side just yet, because the tape usually rips. I tape the bottom to the sides once the basket is erected.
 |
| Go top left to top right, to bottom left to bottom right. |
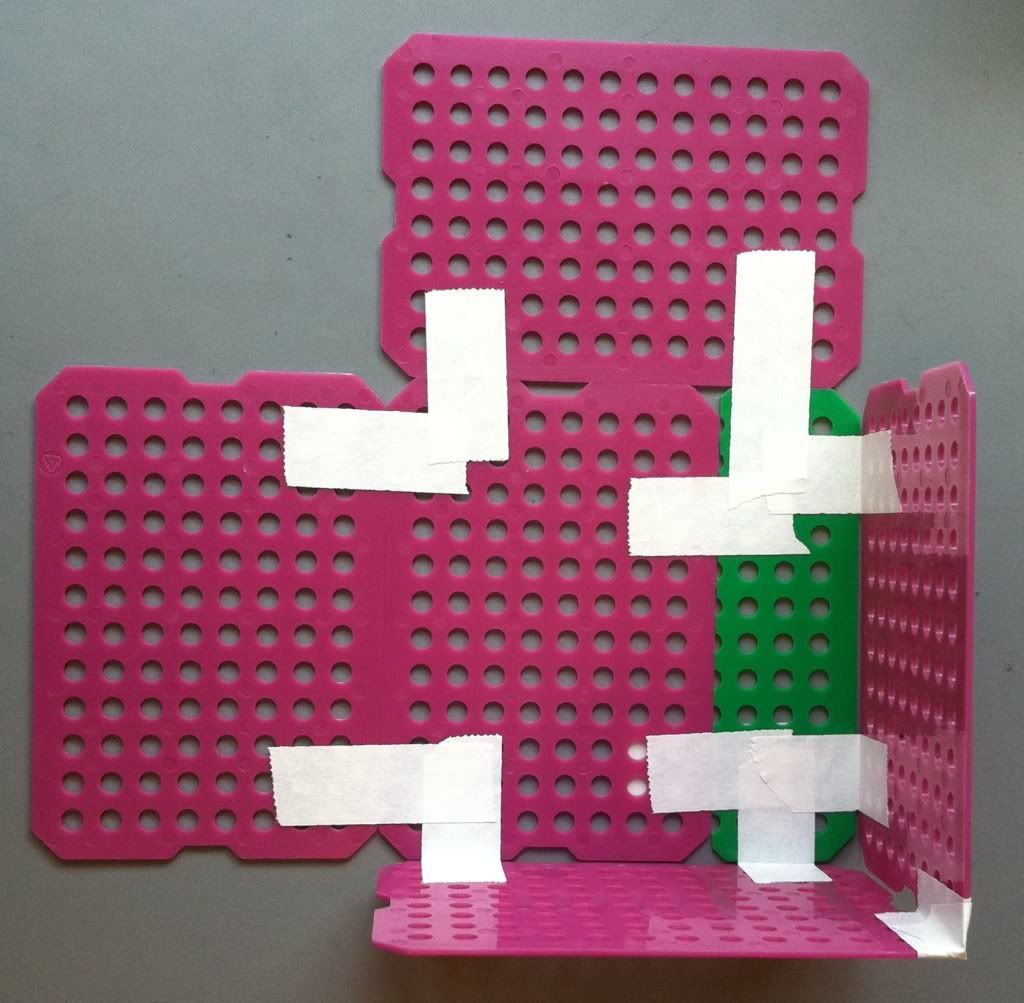 |
| Two sides taped to one another. |
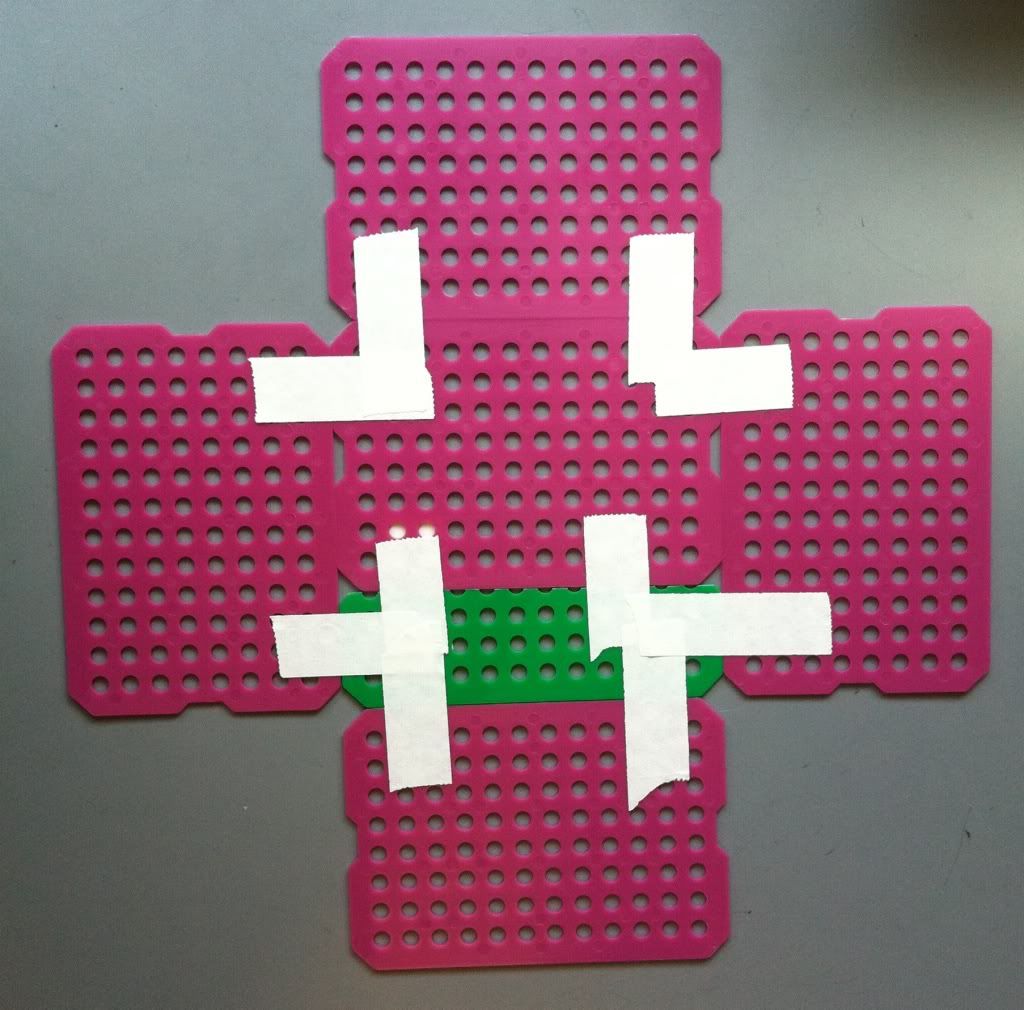
6.) Tape all four of the sides together. Now your basket is (essentially) completed.
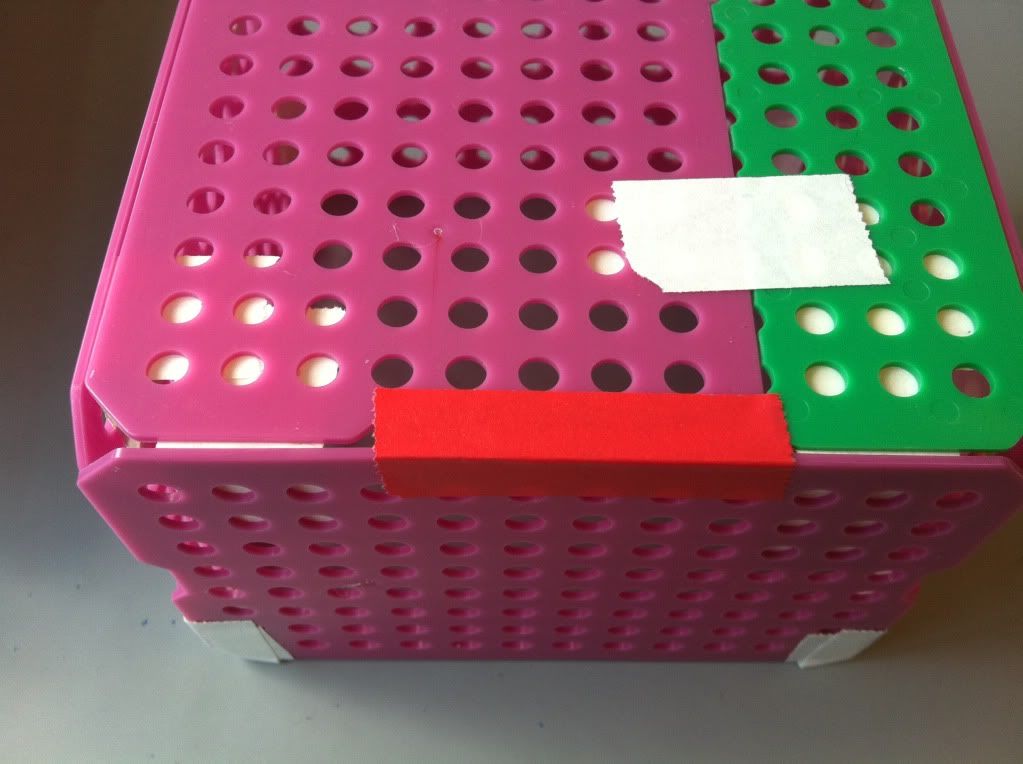
7.) To increase the integrity of the bottom of the basket, a piece of tape along each edge of the bottom taping the bottom to the side of the basket is a good idea. The easiest place to tape is on the outside; if you really want the bottom of the basket to stay attached the sides you can try taping the inside of the bottom to the sides as well. You can also put more take along the corners where the sides of the basket meet each other.
 |
| Looking pretty spiffy! |

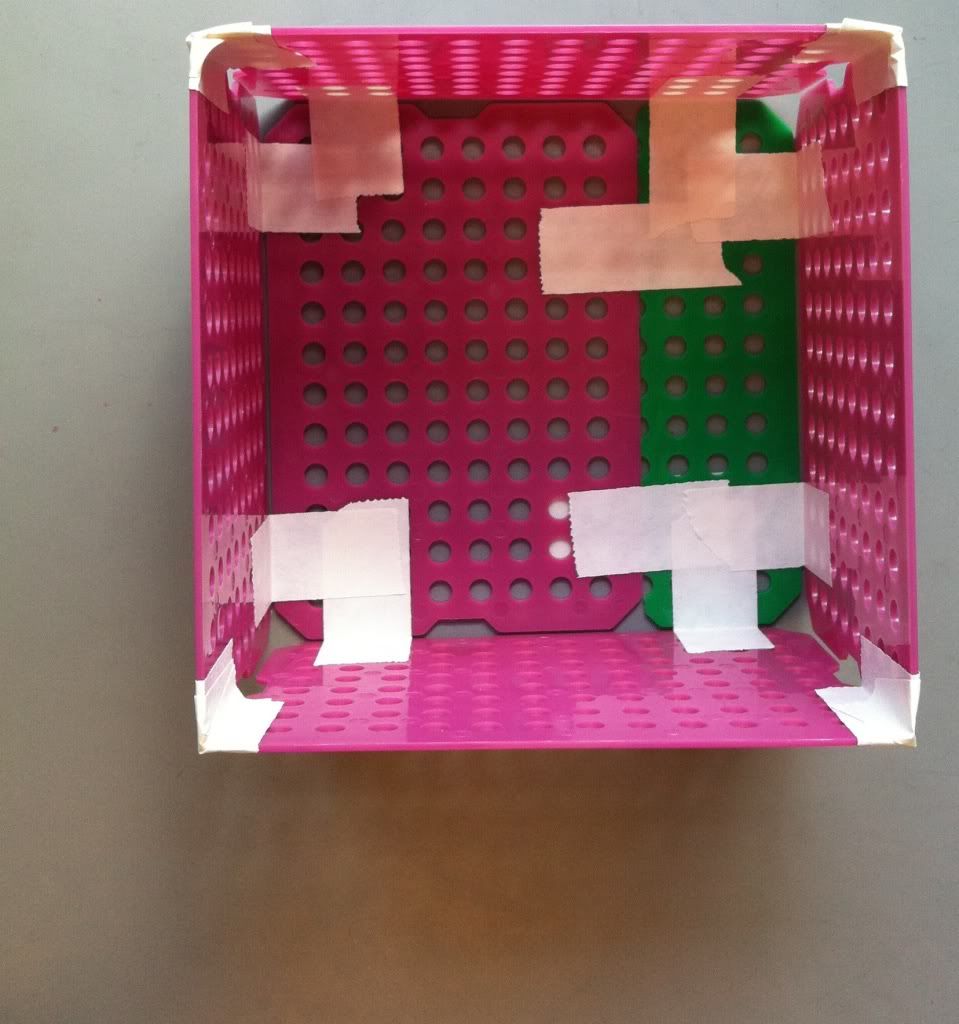
7.) To increase the integrity of the bottom of the basket, a piece of tape along each edge of the bottom taping the bottom to the side of the basket is a good idea. The easiest place to tape is on the outside; if you really want the bottom of the basket to stay attached the sides you can try taping the inside of the bottom to the sides as well. You can also put more take along the corners where the sides of the basket meet each other.
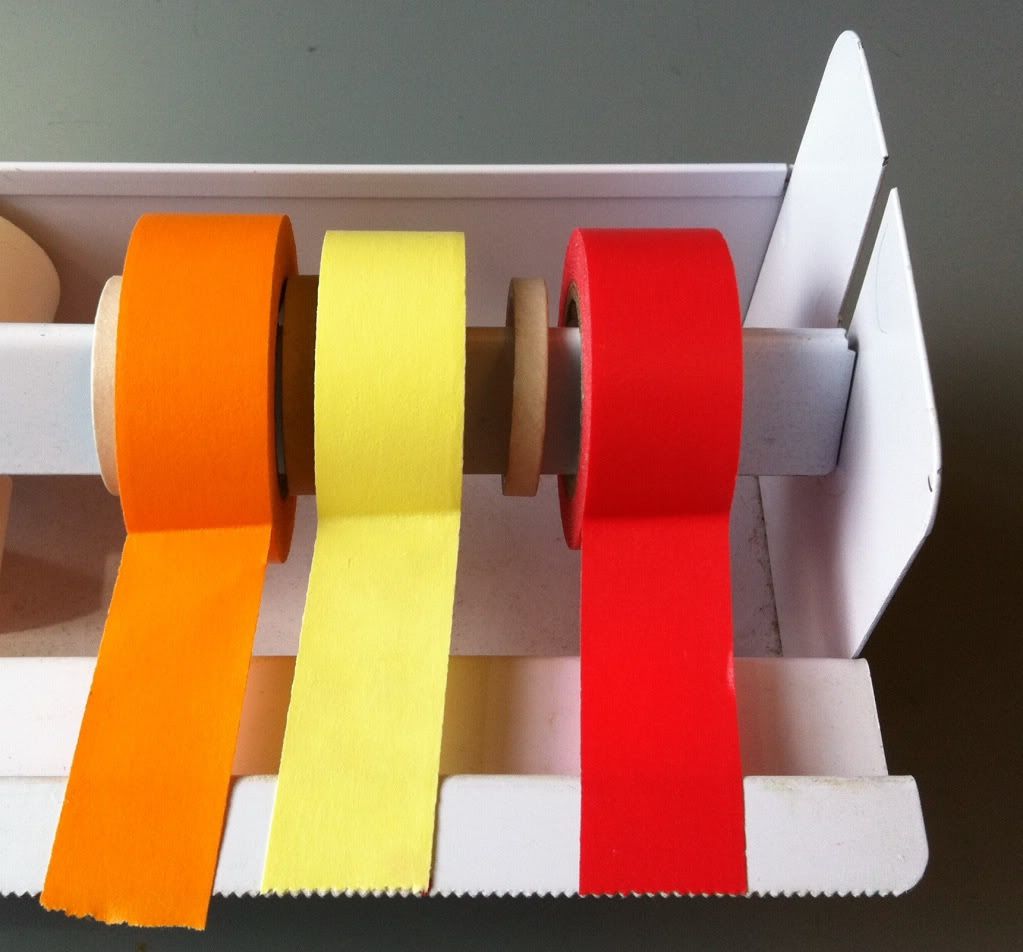
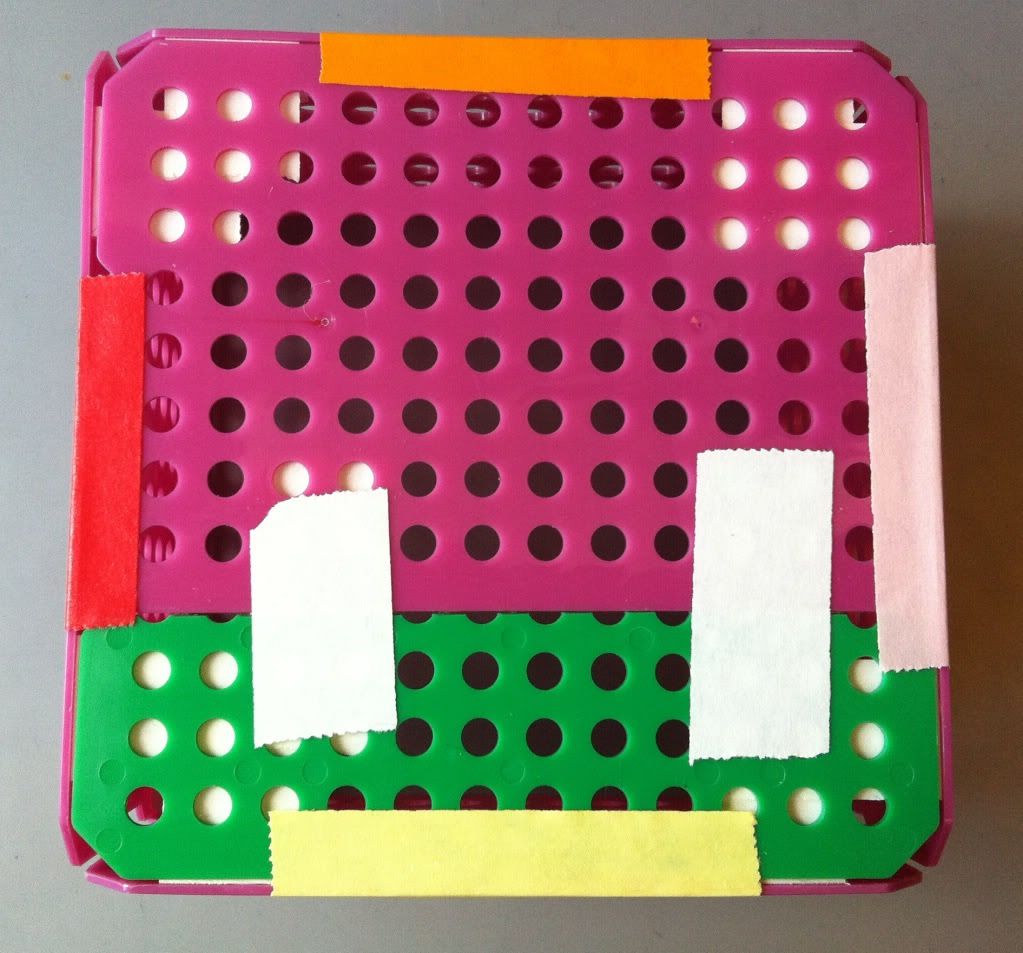 |
| The yellow, red, pink (which isn't very pink), and orange pieces of tape keep the bottom of the basket attached to each side of the basket. |
For a more professional look:
- Try overlapping the plastic sheets to make a customized fit rather than cutting sheets and taping them together. Less tape may look better.
- Cut the edge of your pieces of tape for a flat edge instead of the standard wavy edge you get from the tape dispenser.
- Use only one color of plastic sheet and stick to only one color of tape (I hope you already thought of this.)
> > Feel free to e-mail me with questions and comments.
Thursday, July 7, 2011
Saturday, July 2, 2011
Sup July? SUP SUCCESSFUL TRANSFORMATIONS?
I've been having trouble transforming E. coli cells with my plasmid vector, within which is a small DNA fragment (my NiR terminator) that I will want to restriction cut out. By transforming bacteria with the plasmid vector, I'll make additional copies of the plasmid and be able to freeze and save the plasmid for later use if necessary.
My lab mates and I spent several weeks trying to figure out why our transformations were doing so poorly and why we were receiving such low plasmid yields from transformed bacteria. I myself figured out that one problem was the ampicillin used to make the agar plates upon which we grow our bacteria had degraded over time, and that the antibiotic was not selecting strongly enough to weed out bacteria with plasmids and bacteria without plasmids. This is the reason why we were not getting good plasmid yields and another reason why our bacteria were not growing when transferred from "old" plates to new agar plates with freshly made ampicillin.
We also concluded that the bacteria cells we were transforming were not up to par to yield the results we needed, so we ordered some new transformation kits.
But in order to successfully clone PCR product into a plasmid vector to transform into bacteria, the PCR product needs to be freshly made. In order to get new PCR product, I re-amplified older PCR product in the same reaction I ran before. I ran four different reactions using the PCR DNA in four different DNA concentrations: 1:1, 1:10, 1:100, & 1:1,000 (lanes 2, 3, 4 & 5 in the picture below respectively). This way I can determine which reaction had too much starting DNA and too little. After my reaction, I ran part of it on a gel to see how each reaction went. I definitely got much larger yields in the 1:1 & 1:10 dilutions (there was probably too much DNA even), so I used the second dilution (1:100, lane 4) to clone into the plasmid vector.
Using the vector plasmid, I transformed them into the bacteria and let them grow over night on an agar plate. I then performed a colony screen, which is a PCR reaction using single bacteria colonies to supply the DNA. That PCR reaction yielded the below gel:
What we're seeing in this gel is the molecular ladder at the top and then 10 different colony screen reactions. They're pretty streaky, which is probably because there was a lot of bacterial DNA in each PCR reaction. What I wanted was a single band at around 700 basepairs, which is roughly half way between the two second most right bands on the ladder. As such, lanes 4, 6, 7 & 8 are good candidates for colonies that have my plasmid with the correct insert.
BRB time for the holiday weekend!
My lab mates and I spent several weeks trying to figure out why our transformations were doing so poorly and why we were receiving such low plasmid yields from transformed bacteria. I myself figured out that one problem was the ampicillin used to make the agar plates upon which we grow our bacteria had degraded over time, and that the antibiotic was not selecting strongly enough to weed out bacteria with plasmids and bacteria without plasmids. This is the reason why we were not getting good plasmid yields and another reason why our bacteria were not growing when transferred from "old" plates to new agar plates with freshly made ampicillin.
We also concluded that the bacteria cells we were transforming were not up to par to yield the results we needed, so we ordered some new transformation kits.
But in order to successfully clone PCR product into a plasmid vector to transform into bacteria, the PCR product needs to be freshly made. In order to get new PCR product, I re-amplified older PCR product in the same reaction I ran before. I ran four different reactions using the PCR DNA in four different DNA concentrations: 1:1, 1:10, 1:100, & 1:1,000 (lanes 2, 3, 4 & 5 in the picture below respectively). This way I can determine which reaction had too much starting DNA and too little. After my reaction, I ran part of it on a gel to see how each reaction went. I definitely got much larger yields in the 1:1 & 1:10 dilutions (there was probably too much DNA even), so I used the second dilution (1:100, lane 4) to clone into the plasmid vector.
 |
| I used PCR product from lane 4 to clone into a vector plasmid for the transformation. |
 |
| While this is a slightly messy colony screen gel, several of these colonies should suffice! |
What we're seeing in this gel is the molecular ladder at the top and then 10 different colony screen reactions. They're pretty streaky, which is probably because there was a lot of bacterial DNA in each PCR reaction. What I wanted was a single band at around 700 basepairs, which is roughly half way between the two second most right bands on the ladder. As such, lanes 4, 6, 7 & 8 are good candidates for colonies that have my plasmid with the correct insert.
BRB time for the holiday weekend!
Subscribe to:
Posts (Atom)